
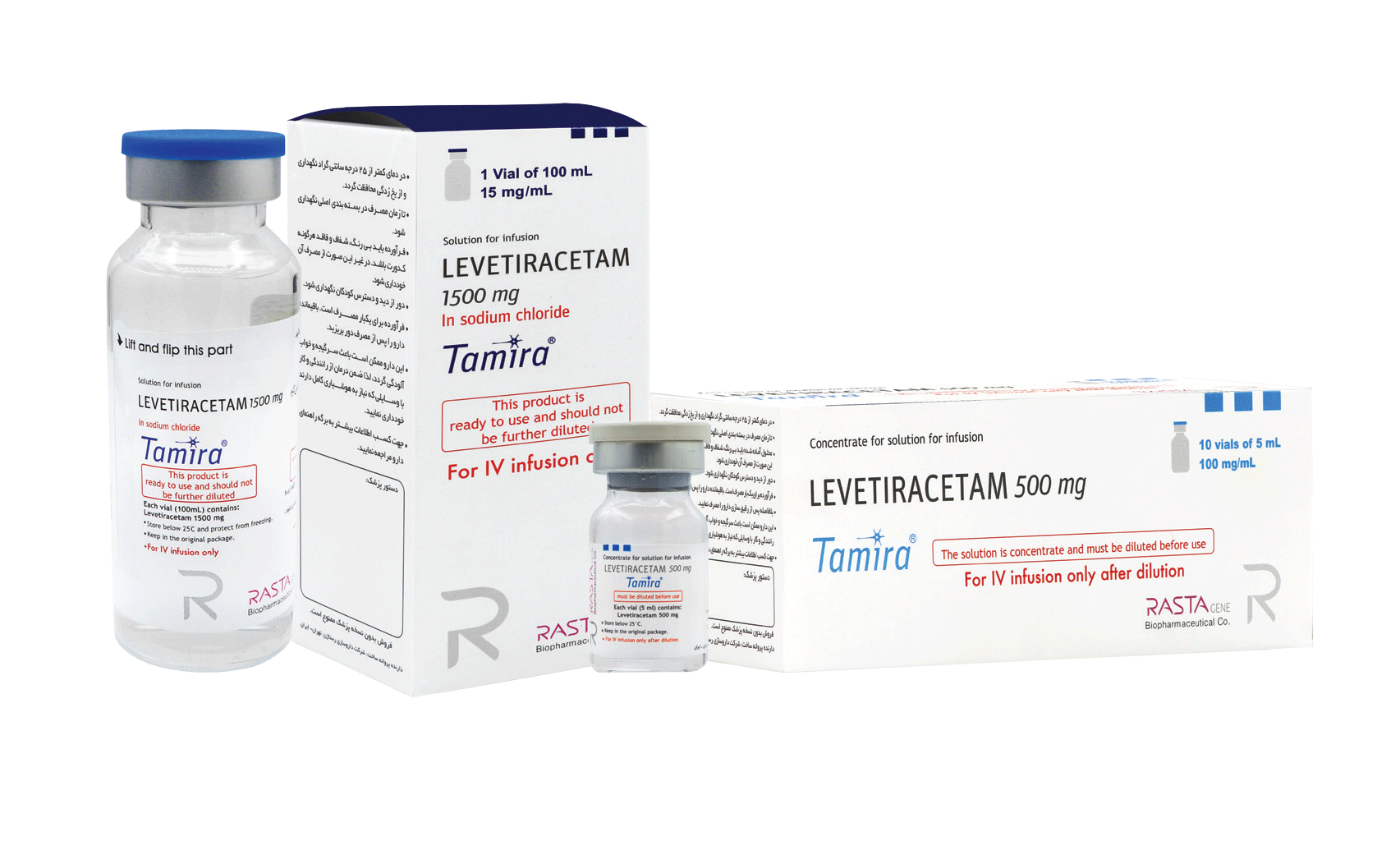
| Partial onset seizure | ||||
| Age range | Age > 16y | 4y < Age < 16y | 6m < Age < 4y | 1m < Age < 6m |
| Initial Dosing | 1000 mg/day, given as twice-daily dosing | 20 mg/kg/day in 2 divided doses | 20 mg/kg/day in 2 divided doses | 14 mg/kg/day in 2 divided doses |
| Maintenance Dosing | 1000 mg/day additional every 2 weeks, MRD*: dose of 3000 mg | 20 mg/kg/day every 2 weeks to RDD* of 60 mg/kg (30 mg/kg twice daily) | 20 mg/kg/day every 2 weeks to RDD* of 50 mg/kg (25 mg/kg twice daily) | 14 mg/kg/day every 2 weeks to RDD* of 42 mg/kg (21 mg/kg twice daily) |
| Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy | ||||
| Initial Dosing | 1000 mg/day, given as twice-daily dosing (500 mg twice daily) | |||
| Maintenance Dosing | 1000 mg/day every 2 weeks to the RDD* of 3000 mg | |||
| Primary Generalized Tonic-Clonic Seizures | ||||
| Age range | Age > 16y | 6y < Age < 16y | ||
| Initial Dosing | 1000 mg/day, given as twice-daily | 20 mg/kg/day in 2 divided doses | ||
| Maintenance Dosing | 1000 mg/day every 2 weeks to RDD* of 3000 mg | 20 mg/kg (10 mg/kg twice daily) every 2 weeks to RDD* of 60 mg/kg (30 mg/kg twice daily) | ||
MRD: Maximum Recommended Dose
RDD: Recommended Daily Dose
Diluents: Sodium chloride 0.9% solution for injection, Ringer's lactate solution for injection, Dextrose 5% solution for injection.
| Group | Creatinine Clearance (mL/min/1.73m²) | Dosage (mg) | Frequency |
| Normal | > 80 | 500 to 1,500 | Every 12 hours |
| Mild | 50 - 80 | 500 to 1,000 | Every 12 hours |
| Moderate | 30 - 50 | 250 to 750 | Every 12 hours |
| Severe | < 30 | 250 to 500 | Every 12 hours |
| ESRD* patients using dialysis | - | 500 to 1,0001 | Every 24 hours1 |
| 1 Following dialysis, a 250 to 500 mg supplemental dose is recommended | |||
ESRD: End-Stage Renal Disease

Address: No. 22, Orfi Shirazi St, North Sheikh Bahaee St, Mollasadra St, Tehran, Iran
Tel: +982188066631-2
Fax: +982189772788
View on map
Rastagene © 2025 - All rights reserved